Detailed description
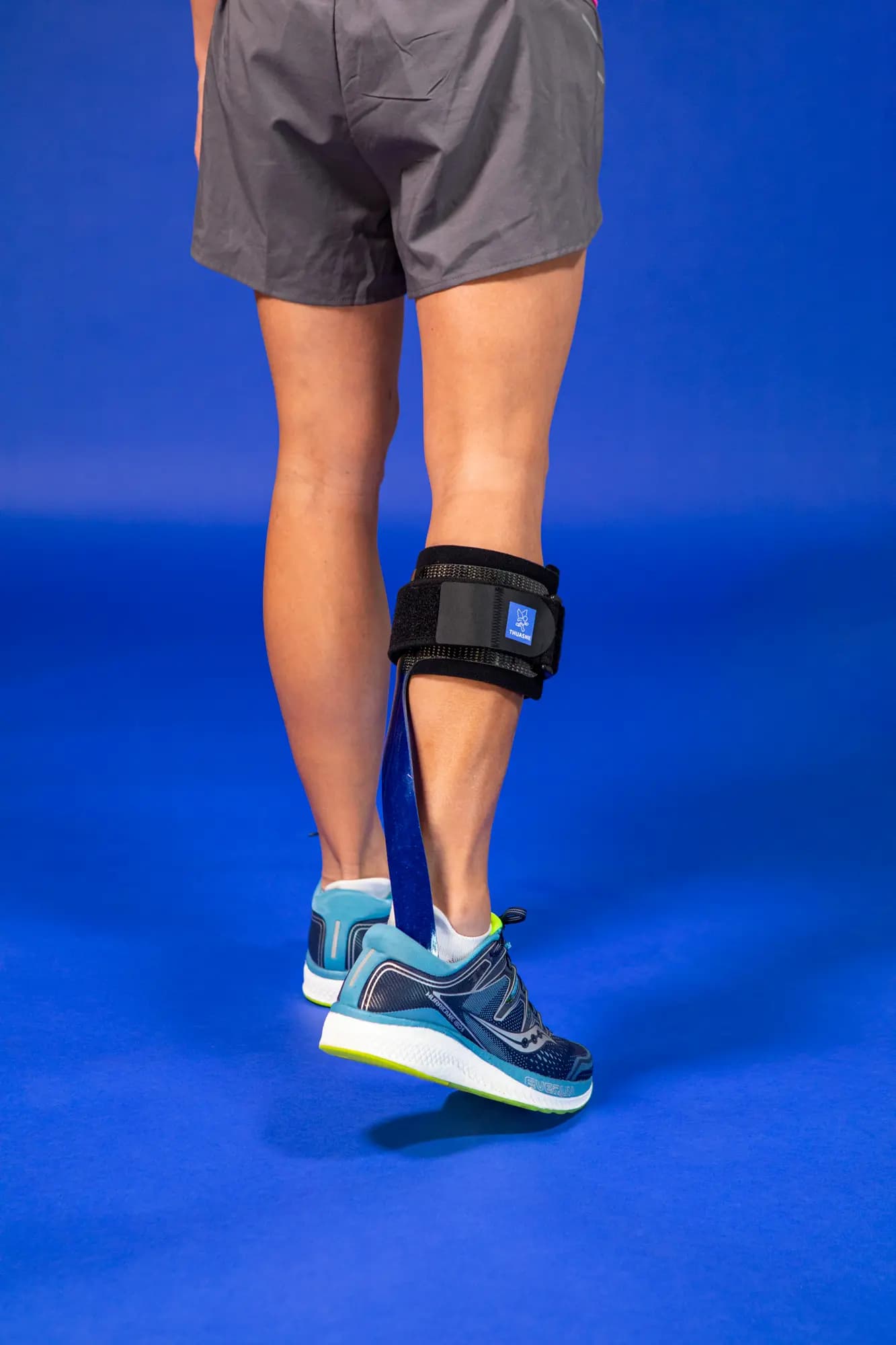
SpryStep® Max is designed to help individuals with foot drop syndrome, a condition that makes it difficult to lift the front part of the foot.
This dynamic ankle-foot orthosis (AFO) is tailored for patients experiencing excessive dorsiflexion, knee instability, or quadricep weakness.
This orthosis is engineered to provide stabilization and support during the stance phase of gait, addressing various biomechanical challenges.
Key benefits of the SpryStep® Max include:
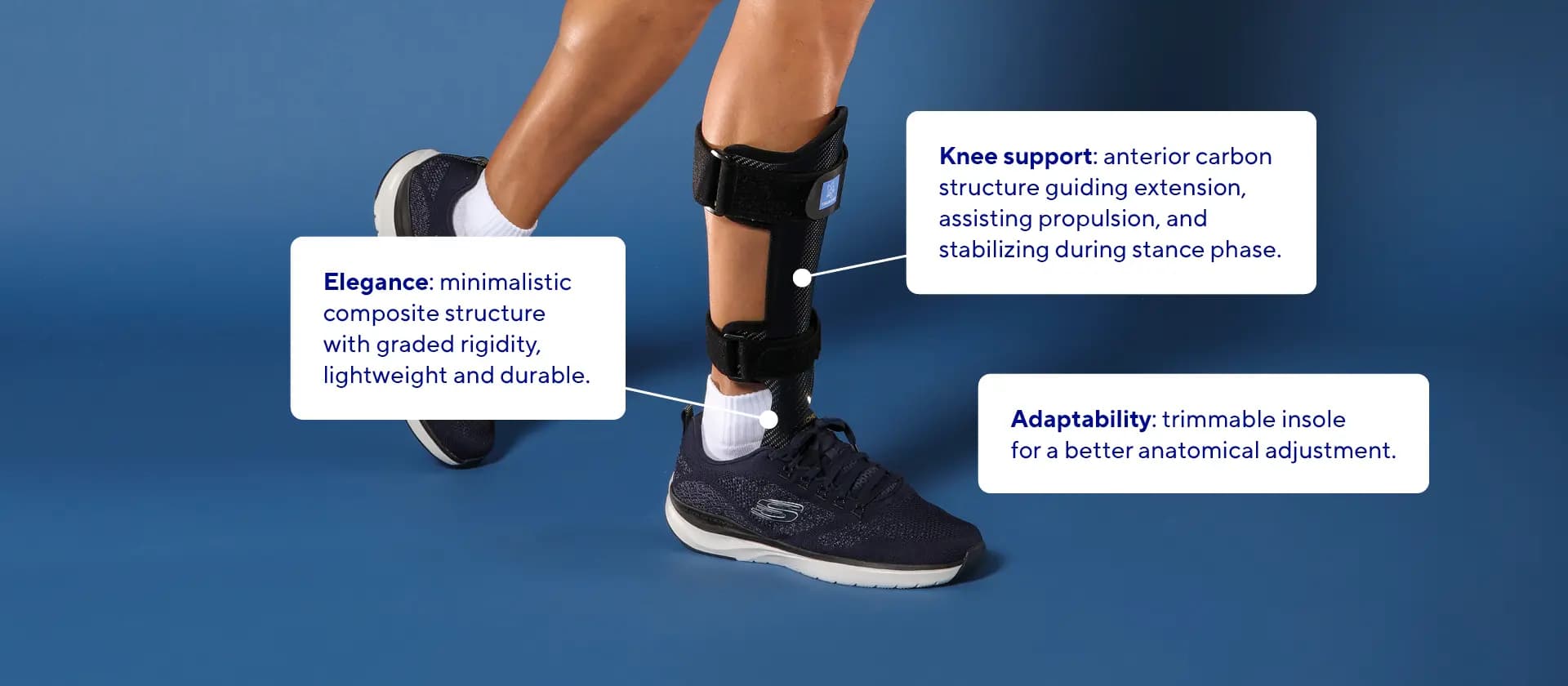
- Strategic Stabilization: The midfoot lateral strut is strategically positioned to optimize gait assistance and stabilization.
- Enhanced Comfort: The anterior shell features a 3D shaped pad with a recessed tibia crest relief zone, ensuring comfort.
- Durable Construction: Made from lightweight, durable composite materials with unique laminated characteristics to prevent delamination and breakage.
- Customizable Fit: The trimmable forefoot section ensures an anatomical adjustment allow to fit for various foot sizes and shapes.
- Patient Compliance: The orthosis's elegant design has been developed to provide both comfort and functionality, with the aim of enhancing patient compliance.
- Versatile Use: Suitable for a wide range of patients, from those with mild to pronounced gait abnormalities.
The SpryStep® Max is available in sizes XS to XL, fitting a broad spectrum of foot sizes. It is offered in both right and left versions to meet individual patient needs. Classified as a Class I medical device, it complies with all relevant regulatory standards.
Cycle-testing independently performed under ISO 10328 Servo-Pneumatic Test System
Patient profile


Innovation
Specifications
Biomechanical deficits of neurological, traumatic or muscular origin.
Weak dorsiflexors.
Footdrop.
Footslap.
Pain in movement towards dorsiflexion.
Mild spasticity of the foot and ankle.
Mild, moderate or severe knee instability during stance phase.
Quadriceps weakness.
Excessive knee flexion during stance phase (secondary to weak
plantarflexors).
Partial foot amputation transmetatarsal or more distal.
SpryStep® Max is intended for patient with lower strength and lower activity
level only (household ambulators/short distances/step too gait).Do not use the product if the diagnosis has not been confirmed.
Do not apply the product in direct contact with broken skin.
Do not use in the event of known allergy to any of the components.
Do not use for patients weighing >120 kg (250 lbs).
Severe loss of sensation in the lower limb.
Open ulcers of the foot, ankle or lower leg.
Moderate to severe oedema of the affected limb.
Moderate to severe spasticity of the foot and ankle.
Triplanar instability.
Plantarflexion contracture.
Moderate to severe foot deformities.
Moderate to severe ankle instabilities.
Running/high impact activities.Size Shoe size Desired length from tip of big toe to heel Size XS 31 - 34 19.5 - 21.5 Size S 34 - 37 21 - 23 Size M 37 - 40 23 - 25.5 Size L 40 - 43 25.5 - 27.5 Size XL 43 - 46 27.5 - 29 Rigid components: carbon fibre - glass fibre - aramid fibre.
Textile components: polyamide - elastane - polyurethane - ethylene vinyl
acetate.
The version of the manual available online is the current version.
Depending on the date of manufacture and place of purchase of the product, this original version of the manual may differ from the version physically present with the product.