Detailed description
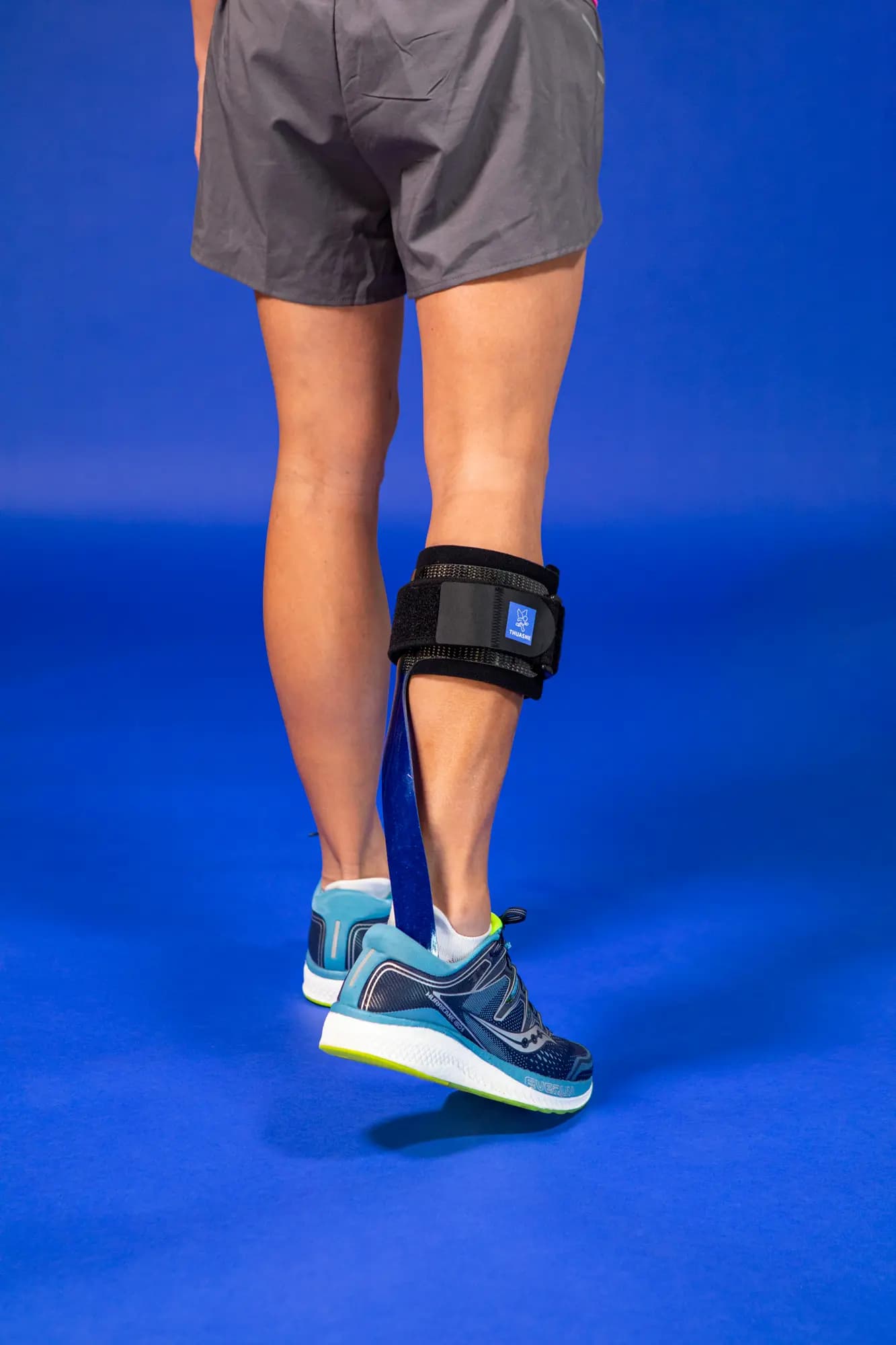
SpryStep® Original is designed to help individuals with foot drop syndrome, a condition that makes it difficult to lift the front part of the foot.
This dynamic ankle-foot orthosis (AFO) provides essential support to the foot, facilitating a more natural walking pattern and reducing the risk of falls.
Key benefits of the SpryStep® Original include:
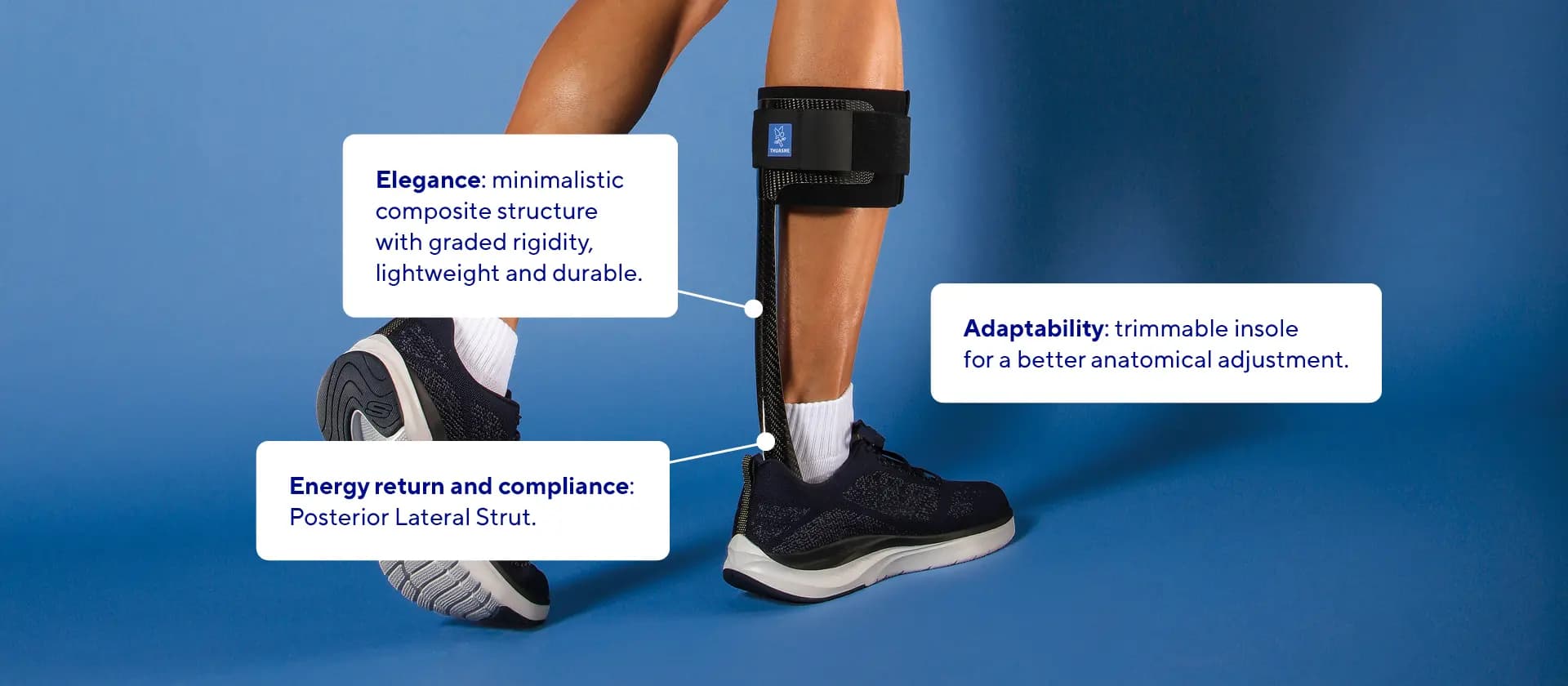
- Lightweight and Durable2: Made from high-quality composite materials, the SpryStep® Original is both lightweight and robust, ensuring long-lasting performance2 and comfort.
- Enhanced Mobility: The specific posterior spiral strut design amplifies energy return1, helping users move more efficiently3.
- Customizable Fit: The trimmable forefoot section ensures a easy fit for various foot sizes and shapes.
- Patient Compliance3: The orthosis's elegant design has been developed to provide both comfort and functionality, with the aim of enhancing patient compliance3.
The SpryStep® Original is available in sizes XS to XL, catering to a wide range of patients. The orthosis is also available in right and left versions to match individual needs. It is classified as a Class I medical device and adheres to all relevant regulatory standards.
1 Internal CE marking data
2 Cycle-testing independently performed under ISO 10328 Servo-Pneumatic Test System
3 Clinically proven: MECASPRY clinical study presented at ISPRM 2022
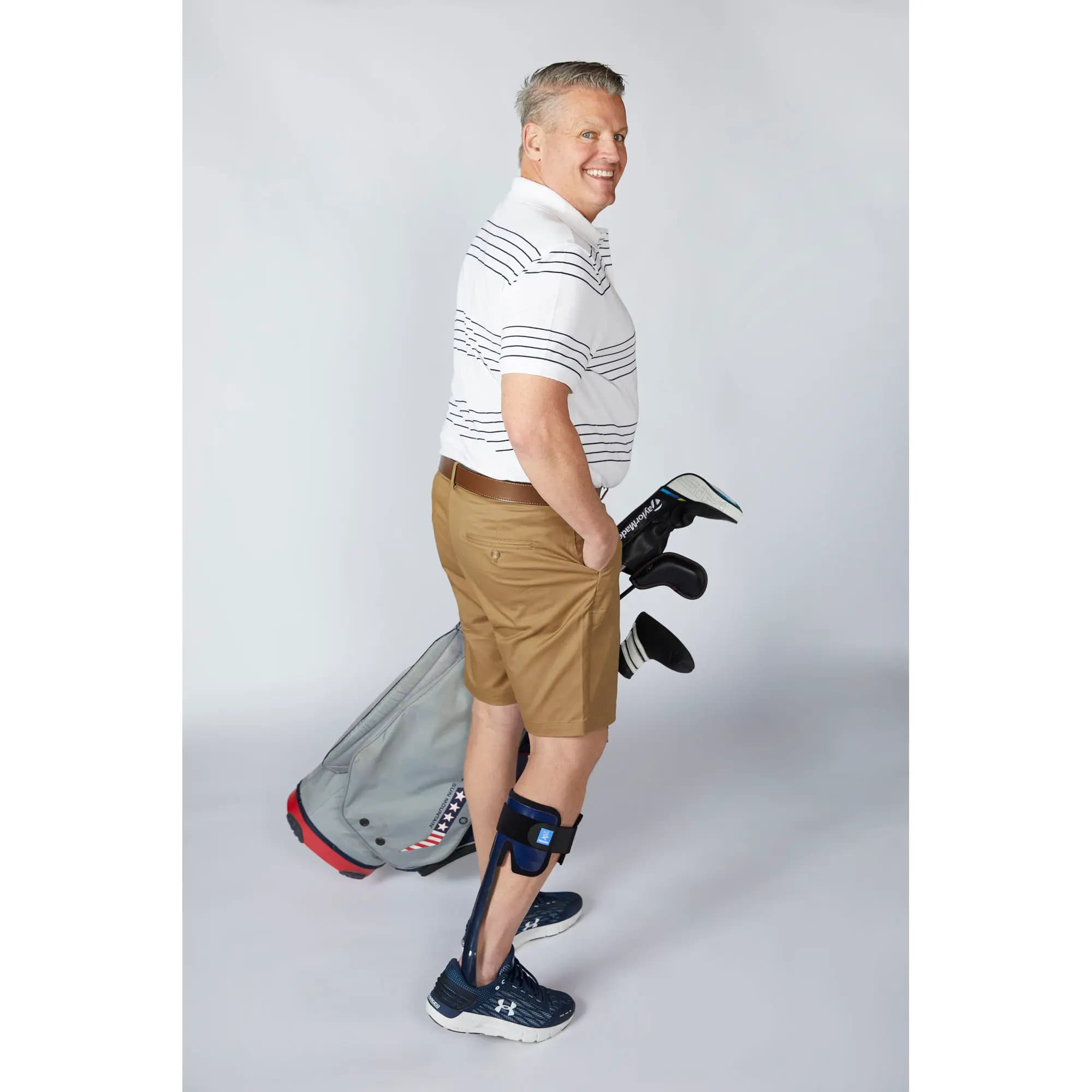
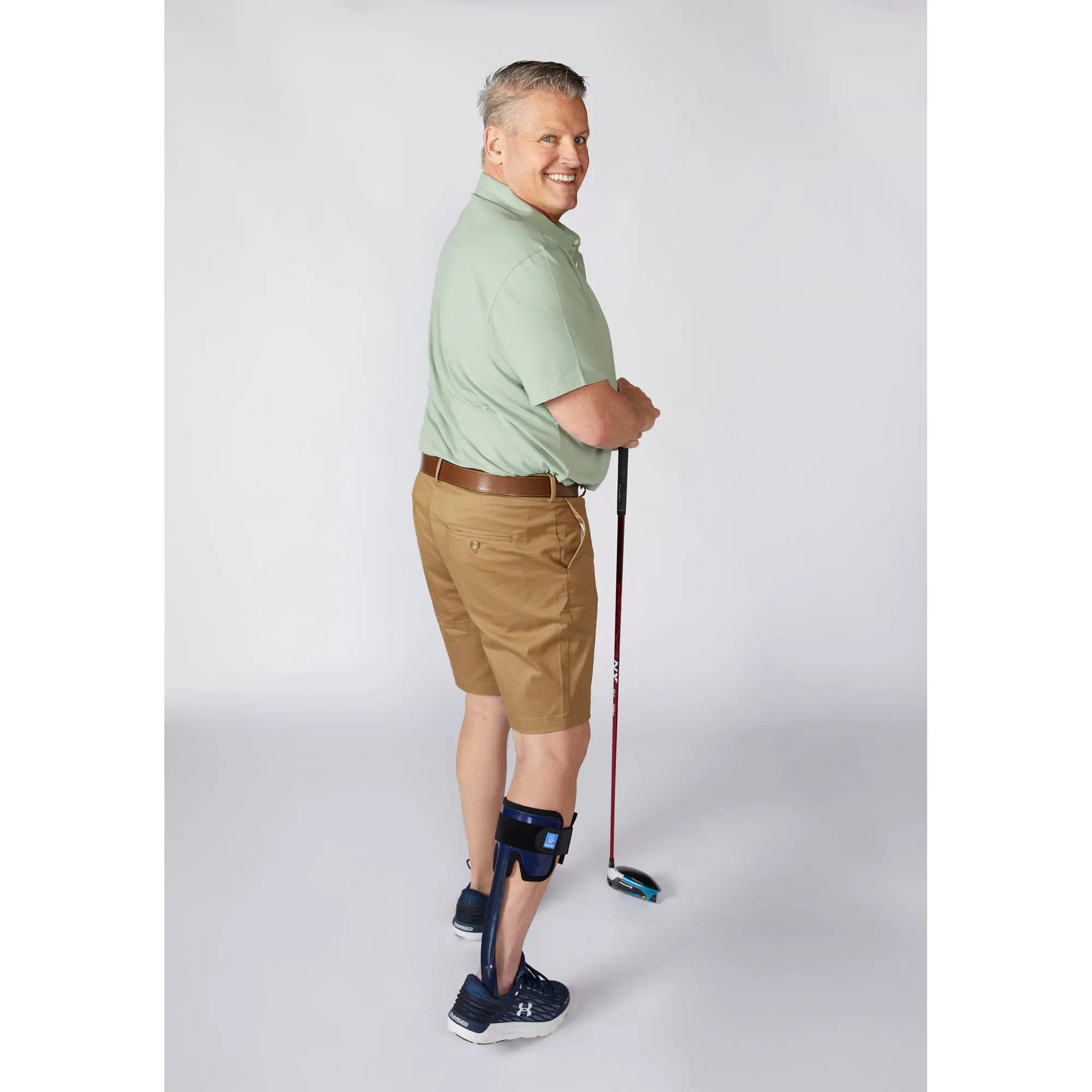
Patient profile


Innovation
Specifications
Biomechanical deficits of neurological, traumatic or muscular origin.
Weak dorsiflexors.
Fatigueable footdrop.
Footdrop.
Footslap.
Plantar flexor strength ≤4.
Mild spasticity of the foot and ankle.
Mild knee instability during stance phase.
Knee hyperextension.
Circumduction.
Vaulting (plantarflexion of the contralateral ankle joint).
High knee gait.
Hip hiking/contralateral trunk bending.Do not use the product if the diagnosis has not been confirmed.
Do not apply the product in direct contact with broken skin.
Do not use in the event of known allergy to any of the components.
Do not use for patients weighing >120 kg (250 lbs).
Severe loss of sensation in the lower limb.
Open ulcers of the foot, ankle or lower leg.
Moderate to severe oedema of the affected limb.
Moderate to severe spasticity of the foot and ankle.
Triplanar instability.
Plantarflexion contracture.
Moderate to severe foot deformities.
Moderate to severe ankle instabilities.
Running/high impact activities.Size Shoe size Size XS 33 - 37 Size S 36 - 39 Size M 38 - 42 Size L 41 - 44 Size XL 44 - 47 Rigid components: carbon fibre - glass fibre - high density polyethylene.
Textile components: polyamide - elastane - polyurethane - ethylene vinyl
acetate.
The version of the manual available online is the current version.
Depending on the date of manufacture and place of purchase of the product, this original version of the manual may differ from the version physically present with the product.