Detailed description
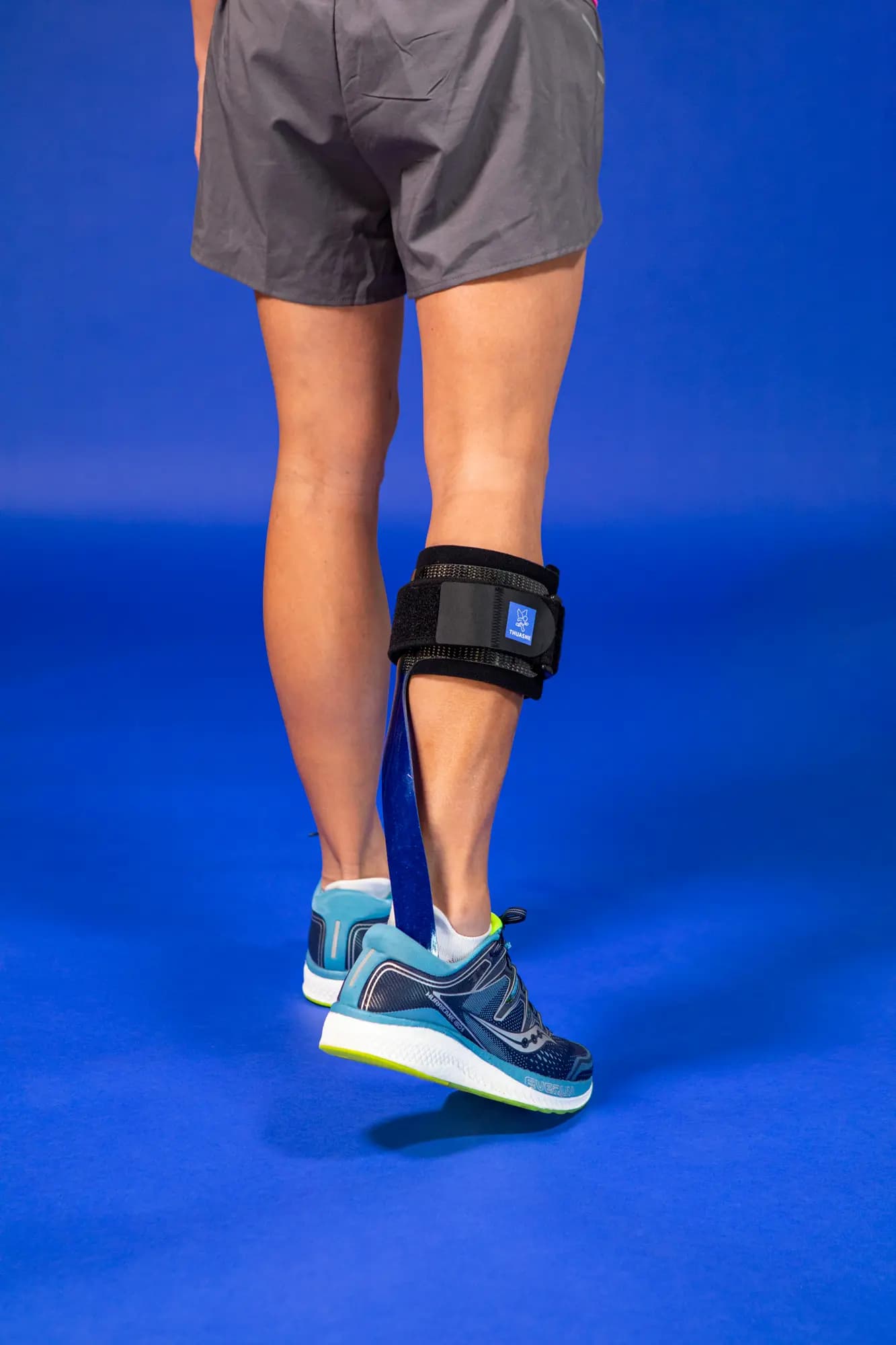
SpryStep® Pediatric is a posterior dynamic ankle-foot orthosis (AFO) crafted to support children with various conditions affecting their mobility, such as foot-drop.
This helps in preventing trips and falls, thus promoting safer movement.
The orthosis's dynamic design allows for a more natural gait.
Key benefits of the SpryStep® Pediatric include:
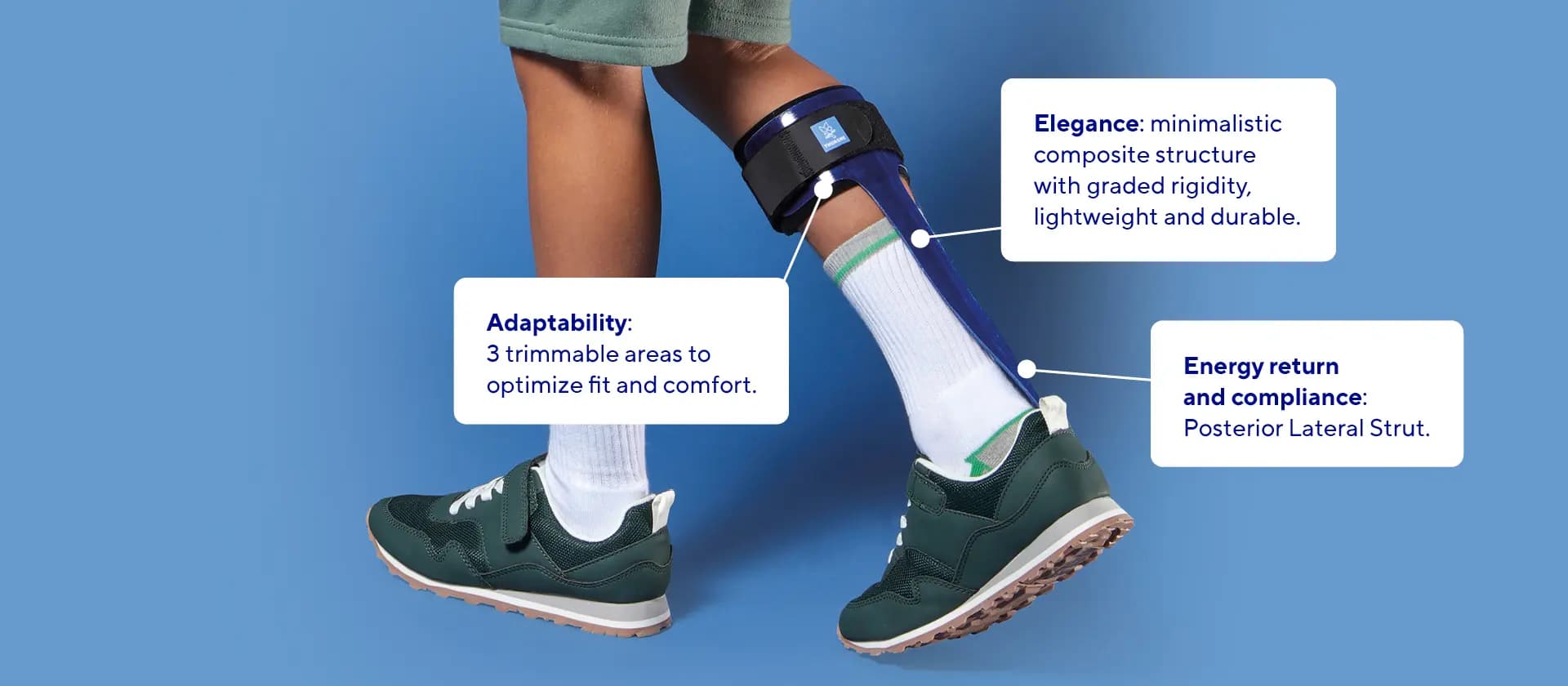
- Lightweight and Durable: Made from high-quality composite materials, the SpryStep® Pediatric is both lightweight and robust, ensuring comfort and ease of use for young patients, allowing them to engage more confidently in daily activities.
- Enhanced Mobility: The unique posterior spiral strut design amplifies energy return, helping children move more efficiently.
- Customizable Fit: 3 trimmable areas to optimize fit and comfort: footplate (toe and medial side of footplate) and calf cuff can easily be adjusted, ensuring an anatomical adjustment that allows for a fit for various foot sizes and shapes.
- Child Compliance: The orthosis's elegant design has been developed to provide both comfort and functionality, with the aim of enhancing child compliance.
The SpryStep® Pediatric is available in sizes XS to XL, catering to a wide range of children. The orthosis is also available in right and left versions to match individual needs. It is classified as a Class I medical device and adheres to all relevant regulatory standards.
Cycle-testing independently performed under ISO 10328 Servo-Pneumatic Test System
Patient profile


Innovation
Specifications
Biomechanical deficits of neurological, traumatic or muscular origin.
Footdrop.
Mild ankle and foot tri-planar instability.
Mild knee hyperextension.
Hypotonia.
High tone.
Delayed standing.
The above may be associated with the following conditions:
Cerebral Palsy.
Poor proprioceptive awareness.
Idiopathic toe walking.
Spina bifida.
Muscular dystrophy.
Hypermobility.
Selective Dorsal Rhizotomy.
Ehlers-Danlos syndrome.Do not use the product if the diagnosis has not been confirmed.
Do not apply the product in direct contact with broken skin.
Do not use in the event of known allergy to any of the components.
Do not use for patients weighing > 60 kg (130 lbs).
Severe loss of sensation in the lower limb.
Open ulcers of the foot, ankle or lower leg.
Moderate to severe oedema of the affected limb.
Moderate to severe spasticity of the foot and ankle.
Triplanar instability.
Plantarflexion contracture.
Moderate to severe foot deformities.
Moderate to severe ankle instabilities.
Moderate to severe fixed ankle varus or valgus conditionsSize Shoe size Foot Length Size XS 17 - 22 9.5 - 14.5 Size S 22 - 26 11 - 16 Size M 26 - 28 12.5 - 17.5 Size L 28 - 31 14 - 19 Size XL 31 - 33 15.5 - 20.5 Rigid components: glass fibre - aramid fibre.
Textile components: polyamide - elastane.
The version of the manual available online is the current version.
Depending on the date of manufacture and place of purchase of the product, this original version of the manual may differ from the version physically present with the product.