Detailed description
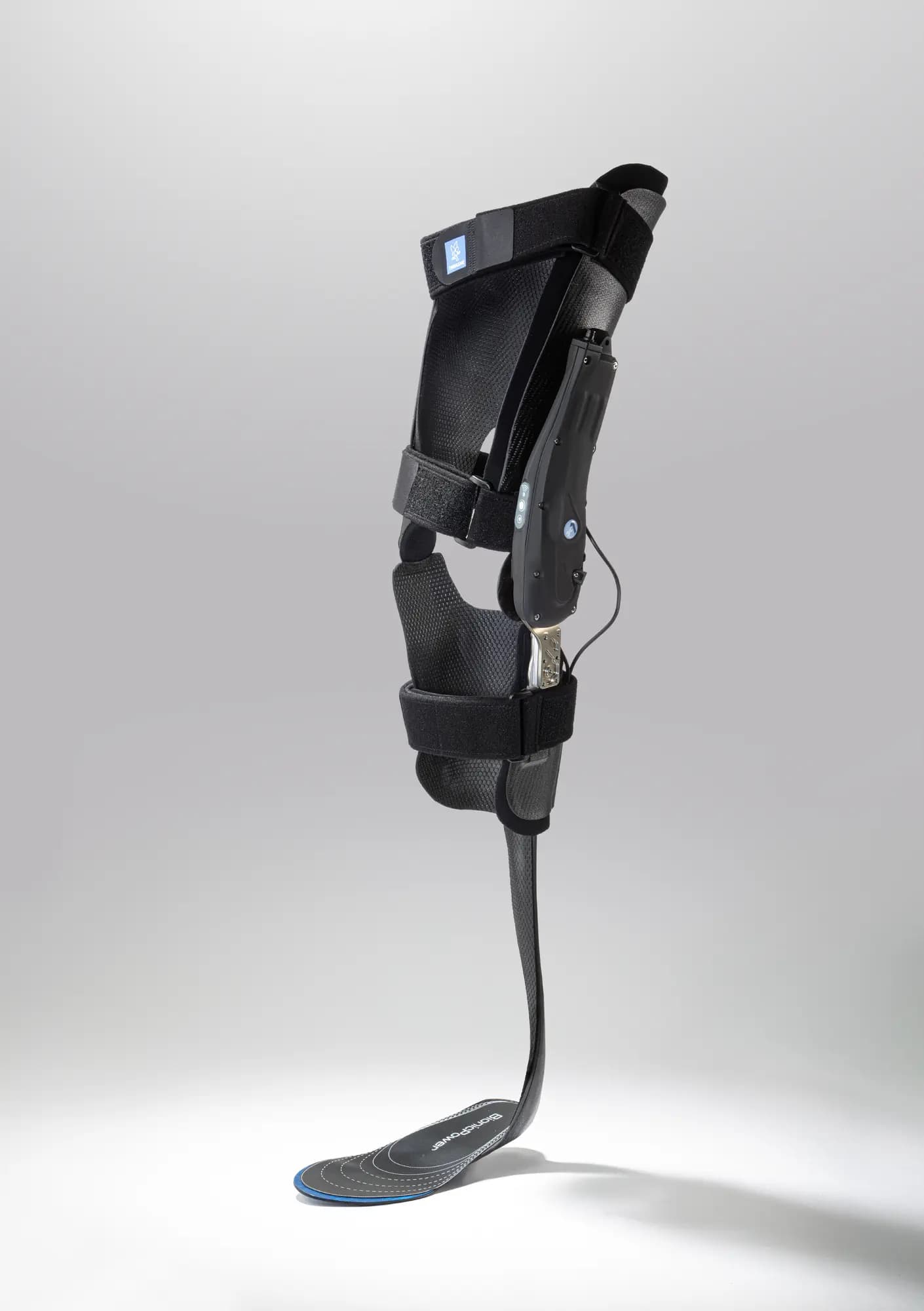
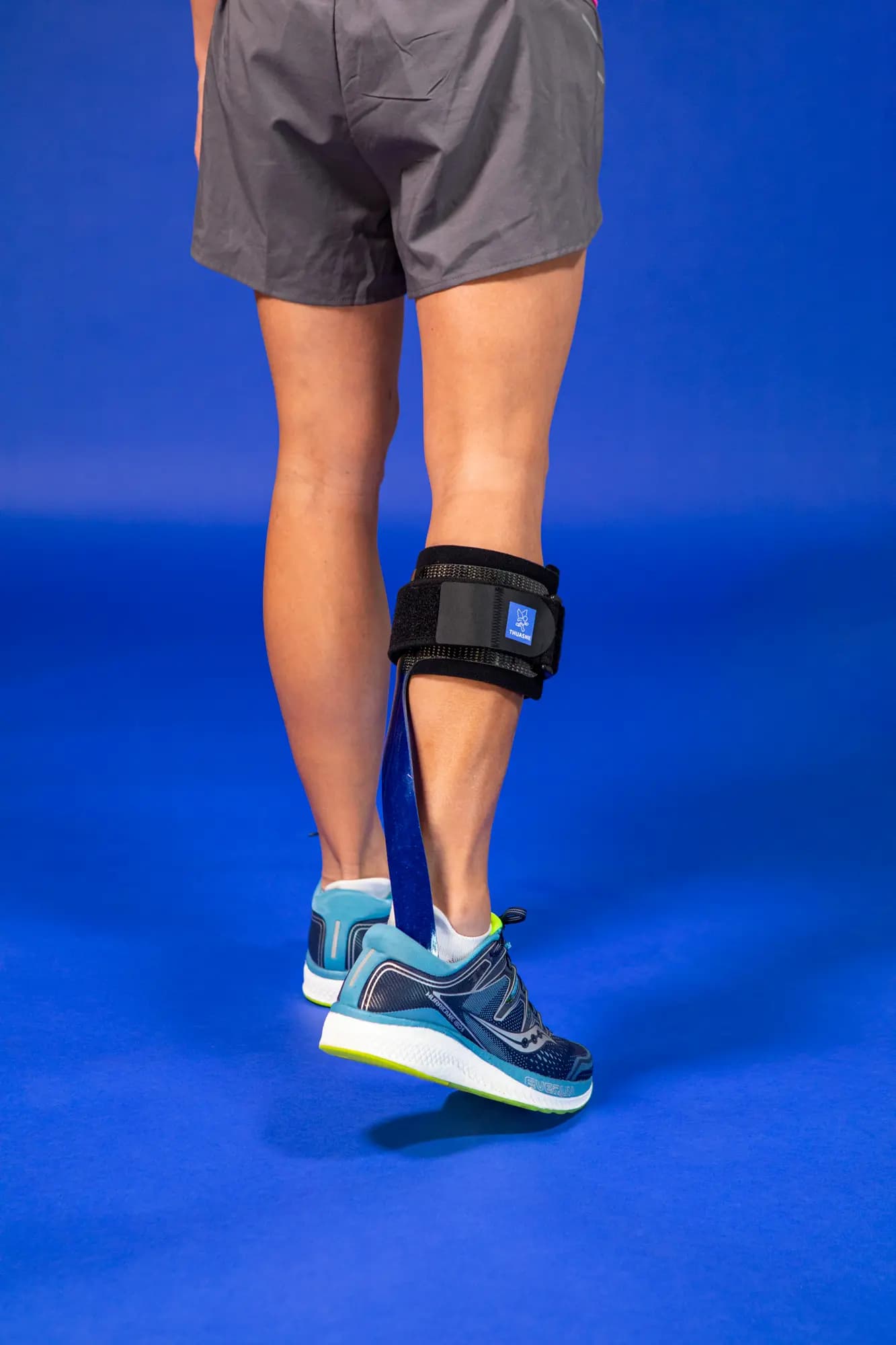
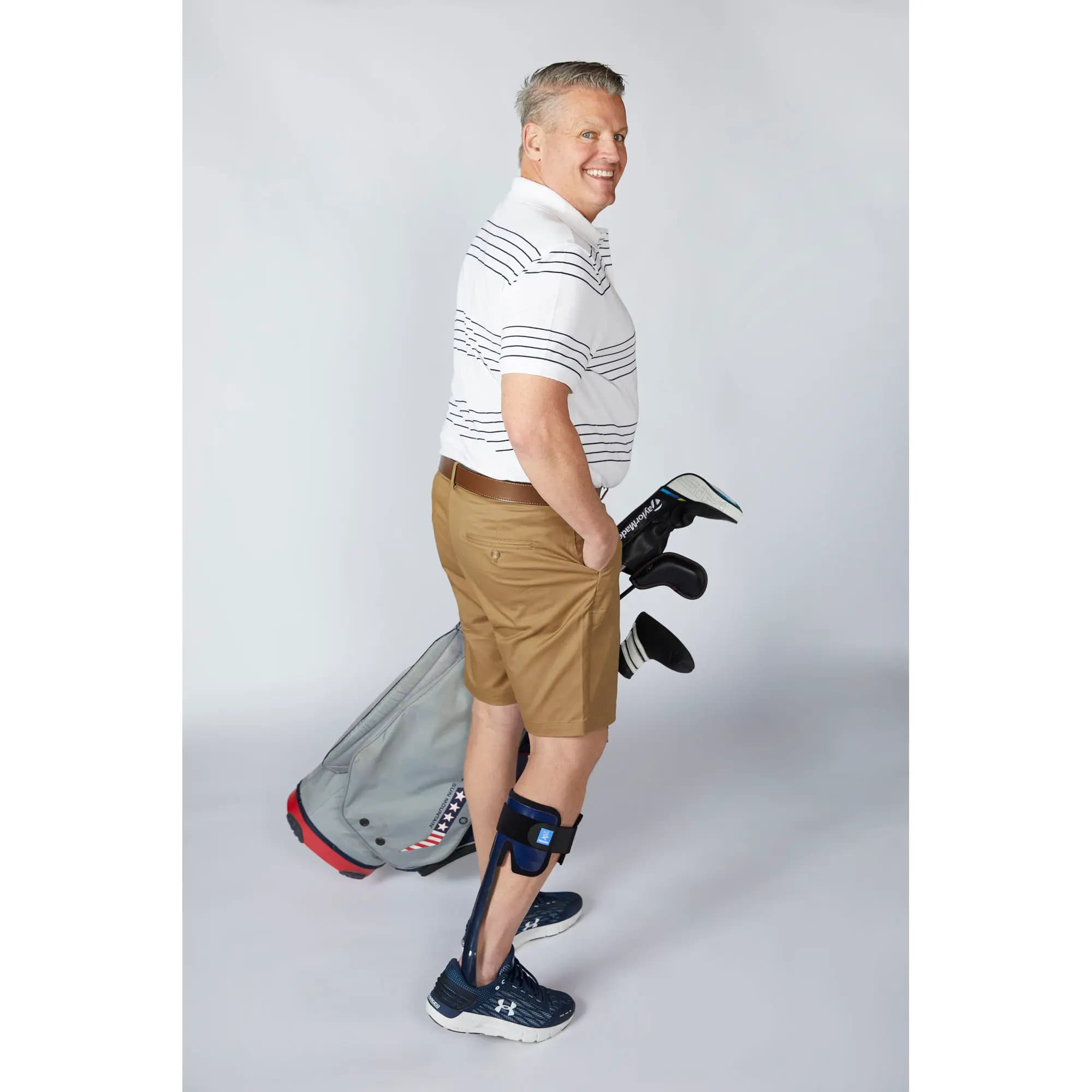
Thanks to its microprocessor-controlled smart system, the SpryStep® Agilik™ KAFO orthosis is a non-invasive mobility solution that detects your movements in real time and assists with knee flexion and extension to improve your gait performance1. This allows you to walk more naturally and improve your posture without limiting your movements2,3.
With its dedicated app, you can track your progress and easily share your data with your healthcare professional for personalised monitoring based on real data.
This exclusive technology was developed as part of a cooperative research and development agreement with the National Institutes of Health (NIH, Bethesda, USA).
- Steinfeldt F, Seifert W, Gunther KP. ‘Modern carbon Fibre orthoses in the management of polio patients a critical evaluation of the functional aspects.’ Z Orthop Ihre Grenzgeb 2003; 141:357 361.
- Devine, Taylor M., et al. "A randomized cross-over study protocol to evaluate long-term gait training with a pediatric robotic exoskeleton outside the clinical setting in children with movement disorders." PloS one 19.7 (2024): e0304087.
- Lerner, Zachary F., Diane L. Damiano, and Thomas C. Bulea. "The effects of exoskeleton assisted knee extension on lower-extremity gait kinematics, kinetics, and muscle activity in children with cerebral palsy." Scientific reports 7.1 (2017): 13512.
Patient profile

Specifications
The product is intended for patients with lower extremity weakness resulting in gait pathology such as, but not limited to crouch gait from a diagnosis of cerebral palsy, muscular dystrophy, spina bifida, or incomplete spinal cord injury or post stroke hemiparesis.
Do not use the product if the diagnosis has not been confirmed.
Do not apply the product in direct contact with broken skin.
Do not use in the event of known allergy to any of the components.
Do not use for patients weighing < 20 kg / 44 lbs and > 135 kg / 300 lbs.
Open ulcers of the foot, ankle or lower leg.
Severe loss of sensation in the lower limb.
Running/jumping/high impact activities on lower limb.
Orthoprosthesis.
Moderate to severe foot deformities (including fixed ankle varus or valgus conditions).
Flexion contracture of more than 20° in the knee and/or hip joint.
Unability to initiate swing phase.
Children below the age of 5 years.
Moderate to severe spasticity of the foot and ankle (as determined by the clinician).Rigid components: carbon fibre - glass fibre - high density polyethylene - ethylene propylene diene monomer - stainless steel.
Textile components: polyamide - elastane - polyurethane - ethylene vinyl acetate.
The version of the manual available online is the current version.
Depending on the date of manufacture and place of purchase of the product, this original version of the manual may differ from the version physically present with the product.